The Louisiana Cancer Research Center (LCRC) , a leading cancer research institute based in New Orleans partnered with Vigyanix to help them implement their clinical trials effectively in Velos eResearch Application. With our extensive knowledge of Velos eResearch application and a strong background in Clinical Trials Implementation, we were confident of positive outcome. We worked closely with LCRC & Tulane hospital, to understand key requirements and to identify the gaps; which when filled, would deliver the highest impact in short-term.
The Challenge
As we started interviewing the clinical research associates, we discovered that there was a huge gap between the expectations the users & sponsors had vs. what Velos eResearch supported out of the box. Clinical research associates (CRAs) were spending 80% of their time in data entry. Since sponsor mandated CRAs to submit the data in a specific template on a paper based form, CRAs would first capture data in Velos eResearch and then enter the same data manually into a PDF form. Not only this process was tedious and time consuming, it drastically increased chances of data errors.
The Solution
With enough information to move forward, we primarily focused on data capturing & reporting since that will enable us to bring maximum efficiency fairly quick.
Velos’ form module provides great flexibility to develop on top of eResearch. Velos provides great guidelines to extend their forms but we chose to take a different approach to enhance the forms. By leveraging cutting edge web technologies, we were able to completely decouple the enhancement code from eResearch’s codebase. This ensured that our code will continue to work with any future releases of Velos as well.
Our goal, of course, was not only wanted to avoid data duplication but rather improve the overall user experience.
[vig_embed_blockquote src=”https://cdn.vigyanix.com/site/wp-content/uploads/blockquote1.png” text=”I have never seen my form work so fast. Amazing!!!” textcolor=”#009bff”]
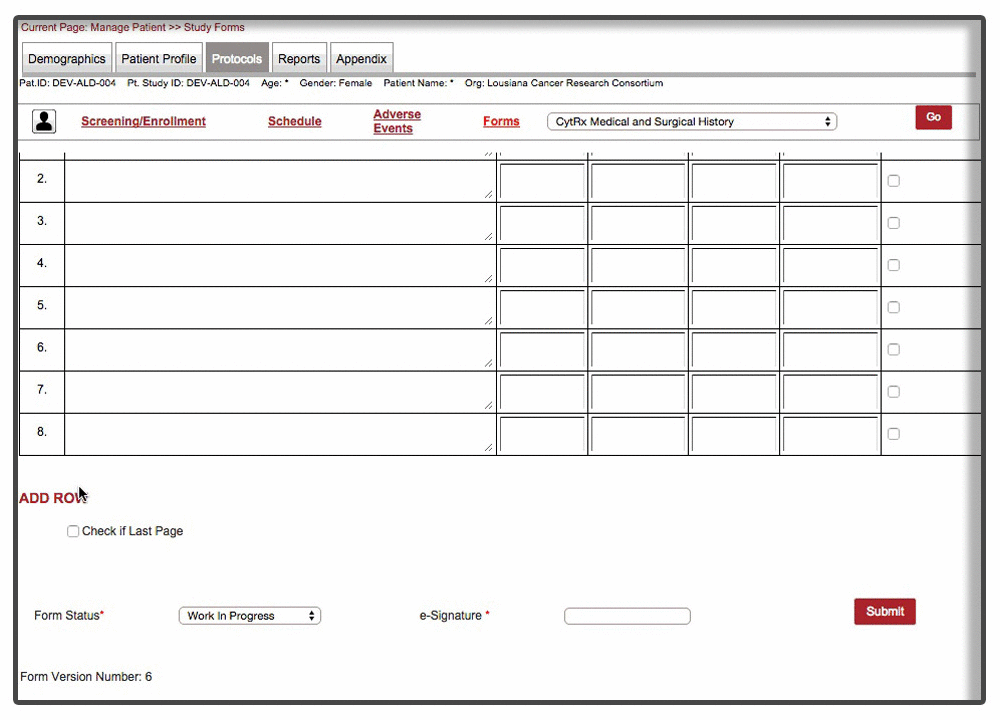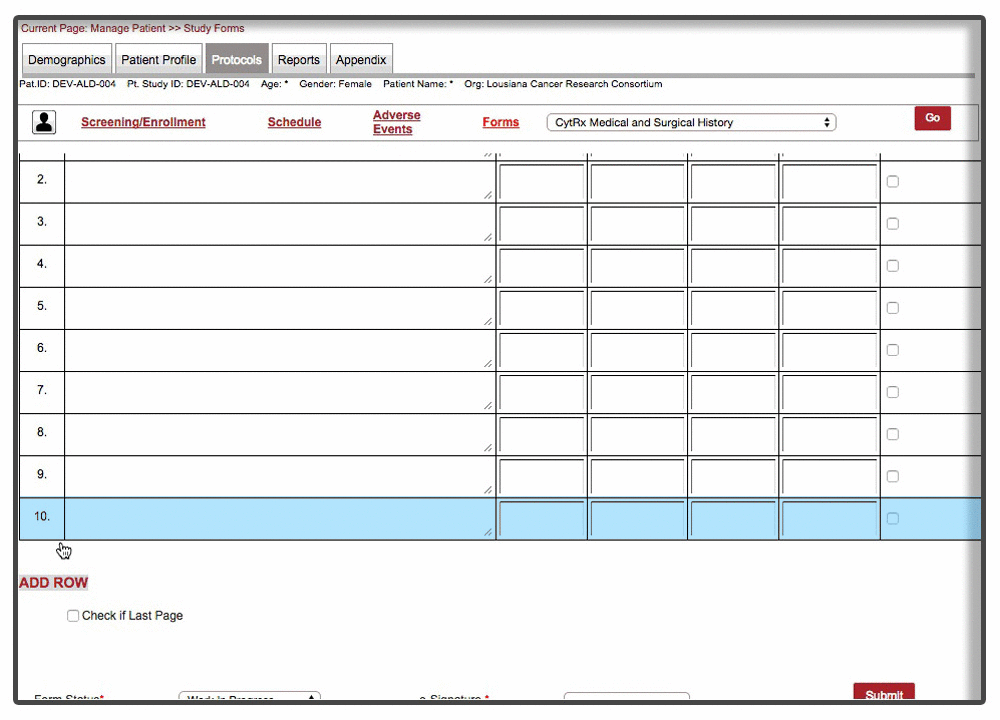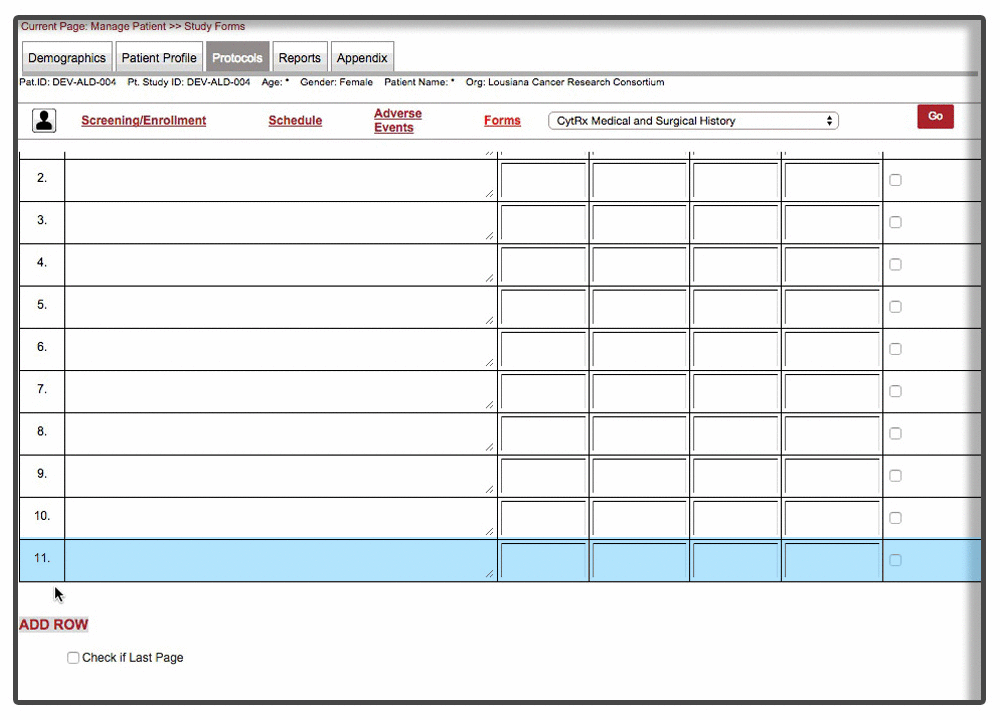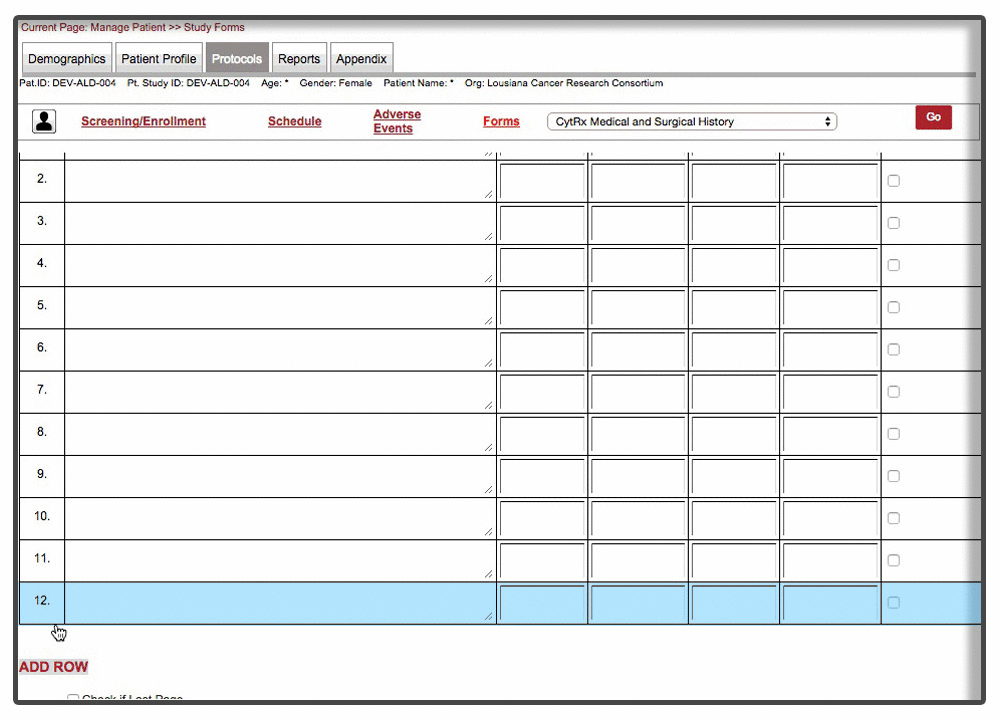
Efficient loading & on-demand rendering : Over 40% of the forms were long and cumbersome. Some of these forms needed to be filled over the entire duration of trial with multiple rows added per patient visit. Because of the scale and complexity involved with form, forms were not responsive and sometime even brought a decent machine to complete halt. We were able to resolve the issue by chunking the form & associated data and load form data on demand. This approach drastically improved the performance of the form with over 70% improvement than the original version of the form.
[vig_embed_blockquote src=”https://cdn.vigyanix.com/site/wp-content/uploads/blockquote1.png” text=”This effectively cuts down 6 clicks for the same process. Tremendous improvement in our workflow. ” textcolor=”#009bff”]
Instant Visit Selection: Another time consuming facet of the process was to switch between different visits during the data entry. Users were spending tremendous amount of time switching between different visits to enter data for the same forms.
Our final implementation enabled users to switch between visits from within the forms in a matter of 2 clicks. A quick demonstration below:
[vig_embed_blockquote src=”https://cdn.vigyanix.com/site/wp-content/uploads/blockquote1.png” text=”Game Changer!!” textcolor=”#009bff”]
Forms Validations: Instant feedback to the user as data being entered was appreciated by the users. Once enabled almost require no further review of the captured data. A huge efficiency gain by the team which not only delivered great experience to the form users but data managers appreciated the “Peace of mind” gained.
[vig_embed_blockquote src=”https://cdn.vigyanix.com/site/wp-content/uploads/blockquote1.png” text=”Even Sponsor could not tell these printouts apart from their own PDF forms.” textcolor=”#009bff”]
Form Reporting: Most importantly, sponsors required the CRAs to enter data in their supplied form’s format. Any data entered in Velos had to be filled manually on those forms and send to sponsor periodically. Our solution was simple here, we just built Velos’ eResearch forms to look exactly like sponsor supplied forms. Every copy printed out of eResearch looked exactly same as sponsor supplied PDF form.